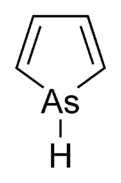
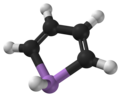
Arsole
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H-Arsole | |||
| Other names
Arsenole
Arsacyclopentadiene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4AsH | |||
| Molar mass | 128.00 g mol−1 | ||
| Related compounds | |||
Related compounds
|
Pyrrole, phosphole, bismole, stibole | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Arsole, also called arsenole[1] or arsacyclopentadiene, is an organoarsenic compound with the formula C4H4AsH. It is classified as a metallole and is isoelectronic to and related to pyrrole except that an arsenic atom is substituted for the nitrogen atom. Whereas the pyrrole molecule is planar, the arsole molecule is not, and the hydrogen atom bonded to arsenic extends out of the molecular plane. Arsole is only moderately aromatic, with about 40% the aromaticity of pyrrole.[2] Arsole itself has not been reported in pure form, but several substituted analogs called arsoles exist. Arsoles and more complex arsole derivatives have similar structure and chemical properties to those of phosphole derivatives. When arsole is fused to a benzene ring, this molecule is called arsindole, or benzarsole.[3]
Nomenclature
[edit]Arsole belongs to the series of heterocyclic pnictogen compounds. The naming of cyclic organoarsenic compounds such as arsole is based on an extension of the Hantzsch–Widman nomenclature system[4] approved by IUPAC, as summarized below:[5]
| Ring size | Unsaturated ring | Saturated ring |
|---|---|---|
| 3 | Arsirene | Arsirane |
| 4 | Arsete | Arsetane |
| 5 | Arsole | Arsolane |
| 6 | Arsinine | Arsinane |
| 7 | Arsepine | Arsepane |
| 8 | Arsocine | Arsocane |
| 9 | Arsonine | Arsonane |
| 10 | Arsecine | Arsecane |
Because of its similarity to the English slang word "arsehole" (in common use outside North America), the name "arsole" has been considered a target of fun, a "silly name",[6][7] and one of several chemical compounds with an unusual name. However, this "silly name" coincidence has also stimulated detailed scientific studies.[2][failed verification][dubious – discuss]
Properties
[edit]| M | d(M-C), Å | d(M-H), Å | α(C-M-C), ° | E, kJ/mol |
|---|---|---|---|---|
| N | 1.37 | 1.01 | 110 | 0 |
| P | 1.81 | 1.425 | 90.5 | 67 |
| As | 1.94 | 1.53 | 86 | 125 |
| Sb | 2.14 | 1.725 | 80.5 | 160 |
| Bi | 2.24 | 1.82 | 78 | 220 |
Arsole itself has not been isolated experimentally yet, but the molecular geometry and electronic configuration of arsole have been studied theoretically. Calculations also addressed properties of simple arsole derivatives, where hydrogen atoms are substituted by other atoms or small hydrocarbon groups, and there are experimental reports on chemical properties of more complex arsole derivatives. The situation is similar for other C4H4MH metalloles where M = P, As, Sb and Bi.
Planarity
[edit]Calculations suggest that whereas pyrrole (C4H4NH) molecule is planar, phosphole (C4H4PH) and heavier metalloles are not, and their pnictogen-bonded hydrogen atom extends out of plane.[9] A similar tendency is predicted for the fluorinated C4F4MH derivatives (M = N, P, As, ..), but the inversion barriers are about 50–100% higher. The planarity is lost even in pyrrole when its nitrogen-bonded hydrogen atom is substituted, e.g., with fluorine. However, the planarity is evaluated in calculation by the energy required to convert between the two configurations where the M-H bond is extending left or right from the molecular plane. However, non-zero (small) value of this energy does not necessarily mean the molecule has low symmetry, because of the possibility of thermal or quantum tunneling between the two configurations.[8]
Aromaticity
[edit]Aromaticity of the arsole manifests itself in delocalization and resonance of its ring electrons. It is closely related to planarity in that the more planar the molecule the stronger its aromaticity.[10] Aromaticity of arsole and its derivatives has been debated for years both from experimental and theoretical points of view. A 2005 review combined with quantum chemical calculations concluded that arsole itself is "moderately" aromatic as its ring current is 40% that of pyrrole, which is known to be aromatic. However, comparable ring current was calculated for cyclopentadiene, which has long been regarded as non-aromatic.[2] Other reports suggest that the aromaticity (and planarity) can vary between arsole derivatives.[9]
Chemical properties (arsole derivatives)
[edit]Chemical properties of arsole derivatives have been studied experimentally; they are similar to those of phosphole and its derivatives.[11] Substitution of all hydrogen atoms in arsole with phenyl groups yields yellow needles of crystalline pentaphenylarsole, which has a melting point of 215 °C. This complex can be prepared, at a yield of 50–93%, by reacting 1,4-diiodo-1,2,3,4-tetraphenylbutadiene[12] or 1,4-dilithio-1,2,3,4-tetraphenylbutadiene with phenylarsenous dichloride (C6H5AsCl2) in ether.
Substituting in this reaction arsenic trichloride for phenylarsenous dichloride yields 1-chloro-2,3,4,5-tetraphenylarsole, which also forms yellow needles but with a lower melting point of 182–184 °C. Pentaphenylarsole can further be oxidized with hydrogen peroxide resulting in yellow crystals with melting point of 252 °C. It can also be reacted with iron pentacarbonyl (Fe(CO)5) in isooctane at 150 °C to yield a solid organoarsenic compound with the formula C34H25As,Fe(CO)3.[11] Reacting pentaphenylarsole with metallic lithium or potassium yields 1,2,3-triphenyl naphthalene.[13]
Reaction of phenylarsenous dichloride with linear diphenyls results in 1,2,5-triphenylarsole (see below), a solid with a melting point of about 170 °C.[14] This compound forms various anions upon treatment with alkali metals.[15]
See also
[edit]- Pyrrole, a nitrogen analog.
- Furan, an oxygen analog.
- Thiophene, a sulfur analog.
- Simple aromatic rings
- Varsol, a petroleum distillate with a boiling range of 150–200 °C.
References
[edit]- ^ Mann 1970: "In English this ring system has frequently named arsenole 'for euphony'."
- ^ a b c M. P. Johansson; J. Juselius (2005). "Arsole Aromaticity Revisited". Lett. Org. Chem. 2 (5): 469–474. doi:10.2174/1570178054405968.
Using quantum chemical methodology, we reinvestigate the aromaticity of the much debated arsole, using the newly developed gauge-including magnetically induced currents (GIMIC) method. GIMIC provides a quantitative measure of the induced ring current strength, showing arsole to be moderately aromatic.
- ^ A. Muranaka; S. Yasuike; C.-Y. Liu; J. Kurita; N. Kakusawa; T. Tsuchiya; M. Okuda; N. Kobayashi; Y. Matsumoto; K. Yoshida; D. Hashizume; M. Uchiyama (2009). "Effect of Periodic Replacement of the Heteroatom on the Spectroscopic Properties of Indole and Benzofuran Derivatives". J. Phys. Chem. A. 113 (2): 464–473. doi:10.1021/jp8079843. PMID 19099440.
- ^ "Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles Archived 2017-09-08 at the Wayback Machine" at IUPAC, retrieved 29 Sept 2008
- ^ Nicholas C. Norman (1998). Chemistry of arsenic, antimony, and bismuth. Springer. p. 235. ISBN 978-0-7514-0389-3. Retrieved 15 March 2011.
- ^ Richard Watson Todd (25 May 2007). Much ado about English: up and down the bizarre byways of a fascinating language. Nicholas Brealey Publishing. p. 138. ISBN 978-1-85788-372-5. Retrieved 15 March 2011.
- ^ Paul W May, Molecules with Silly or Unusual Names, publ. 2008 Imperial College Press, ISBN 978-1-84816-207-5(pbk). See also the Web page "Molecules with Silly or Unusual Names Archived 2009-09-07 at the Wayback Machine" at the School of Chemistry, University of Bristol, (retrieved 29 Sept 2008)
- ^ a b Pelzer, Silke; Wichmann, Karin; Wesendrup, Ralf; Schwerdtfeger, Peter (2002). "Trends in Inversion Barriers IV. The Group 15 Analogous of Pyrrole". The Journal of Physical Chemistry A. 106 (26): 6387–6394. doi:10.1021/jp0203494.
- ^ a b Tadeusz Marek Krygowski; Michal K. Cyrański; M. Agostinha R. Matos (2009). Aromaticity in Heterocyclic Compounds. Springer. pp. 47–. ISBN 978-3-540-68329-2. Retrieved 21 March 2011.
- ^ Pelloni, Stefano; Lazzeretti, Paolo (2007). "Magnetotropicity of phosphole and its arsenic analogue". Theoretical Chemistry Accounts. 118: 89–97. doi:10.1007/s00214-007-0247-0. S2CID 97528113.
- ^ a b Mann, Frederick George (1970). The heterocyclic derivatives of phosphorus, arsenic, antimony, and bismuth. John Wiley and Sons. pp. 357–360. ISBN 978-0-471-37489-3. Archived from the original on 13 June 2014. Retrieved 21 March 2011.
- ^ Braye, E. H.; Hubel, W.; Caplier, I. (1961). "New Unsaturated Heterocyclic Systems. I". Journal of the American Chemical Society. 83 (21): 4406–4413. doi:10.1021/ja01482a026.
- ^ C. W. Bird; Gordon William Henry Cheeseman (31 December 1973). Aromatic and Heteroatomic Chemistry. Royal Society of Chemistry. pp. 23–. ISBN 978-0-85186-753-3. Archived from the original on 13 June 2014. Retrieved 23 March 2011.
- ^ Gottfried Märkl & Hagen Hauptmann (1972). "Unusual Substitution in an Arsole Ring" (PDF). Angewandte Chemie International Edition in English. 11 (5): 441. doi:10.1002/anie.197204411. Archived (PDF) from the original on 2011-05-24. Retrieved 2011-03-23.
- ^ Märkl, G (1983). "Synthese von 1-phenyl-2,5-diaryl(dialkyl)-arsolen; umsetzung der arsole mit alkalimetallen und lithiumorganylen". Journal of Organometallic Chemistry. 249 (2): 335–363. doi:10.1016/S0022-328X(00)99433-6.